Public Procurement Law in the Context of Medical Cannabis in Germany
In this Expert Focus article, Karin Deichmann, René Kieselmann and Margret Knitter of SKW Schwarz provide an insight into the German public procurement process in relation to medical cannabis.
Karin Deichmann
René Kieselmann
Margret Knitter
Introduction
With regard to the procurement of cannabis, public procurement law in Germany is of great importance. The background is that no explicit licences are issued for the free cultivation of medical cannabis. Rather, supply contracts with private suppliers are concluded. These contracts define the terms of time and quantity. In April and May 2019, the Federal Institute for Drugs and Medical Devices (BfArM) awarded the contract for cultivation, harvesting and processing of cannabis for medicinal purposes for a total of 10,400kg over four years. A contractor for the distribution of medical cannabis was selected in 2020 in another European tender process.
Actors in cannabis procurement
(Cannabis market players) need to familiarise themselves with the rules for tendering in order to successfully compete in the market.
The Cannabis Agency (Cannabis Agentur) established at the BfArM is responsible for the controlled cultivation, harvesting, processing, quality testing, storage, packaging and dispensing to pharmacies of medicinal cannabis flowers in Germany. As a consequence of this actual regulation, the German public procurement law (eg, Act against Restraints of Competition – GWB) applies to the respective selection process.
Cannabis is cultivated by companies that were selected in a Europe-wide tendering procedure (platform: https://ted.europa.eu/) and commissioned by the cannabis agency. In this procedure, all pharmaceutical and narcotic legal requirements are taken into account.
Thus, Germany does not actually issue cannabis cultivation licences (eg, as concession to sell on a market to an undefined number of customers).
Besides that, German public procurement law governs the distribution of medical cannabis. This tender also fell within the competence of the BfArM. However, these public procurement procedures were less complex and less controversial, as there were no pending review procedures.
For some years now, health insurance funds have been considered “public contracting authorities” and are thus subject to the rules of public procurement law. Services in the field of medical cannabis must therefore also be put out to public tender when the state is responsible. In the context of the upcoming liberalisation, competencies and responsibilities may change.
For cannabis market players, this means that they need to familiarise themselves with the rules for tendering in order to successfully compete in the market.
Proceedings before the public procurement tribunals
The cultivation of medical cannabis was very controversial before the public procurement tribunals. Various proceedings of bidders involved in the procedure were pending before the Federal Procurement Chamber (Federal Public Procurement Tribunal, Decisions of 01.08.2017, 09.08.2017, 25.10.2017, 13.11.2017 and 19.10.2018) and, in the second instance, before the Higher Regional Court of Duesseldorf. As a result, the planned award has been significantly delayed.
In the review proceedings, various problem areas under public procurement law were addressed.
Since cannabis was not legally grown commercially in Germany until recently, German applicants lack experience. In this context, the applicant company in procedure VK 1-69/17 complained that the deadline was too short to be able to name a subcontractor with corresponding experience in the field, as required. In proceedings VK 1-77/17, there was a dispute as to whether the company bidding for the contract submitted a declaration of commitment from a foreign company that met the requirements and whether it could prove that it had access to the company's experience in cannabis cultivation.
The award procedure was then stopped by several second-instance decisions.
The Duesseldorf Higher Regional Court confirmed on 28.03.2018 that even under the new public procurement law, a subsequent correction of proof of suitability is unlawful. The Public Procurement Act (Section 56 (2) VgV – Vergabeverordnung) does provide that contracting authorities may request bidders to submit, complete or correct missing, incomplete or incorrect suitability-related documents. However, this does not mean that bidders may also improve the content of their proof of suitability in the context of a subsequent request.
On the same day, the Duesseldorf Higher Regional Court ruled that the time limits under Section 20 VgV (21 days) were too short.
Tender of health insurance
With the notice of 06.12.2021 (Number: 2021/S 236-621151), various health insurance plans advertised the conclusion of a non-exclusive discount contract (known as an Open-House-Model) according to Section 130a (8) SGB V for the active substance medicinal cannabis extracts.
It was, however, not a matter of awarding public contracts within the meaning of Directive 2014/24/EU or Section 97 GWB. Any pharmaceutical entrepreneur pursuant to Section 4 (18) of the German Medicines Act (AMG) may become a contracting party. According to the case law of the ECJ, such authorisation procedures are not necessarily subject to public procurement law, as no competition takes place due to the lack of a selection decision. Rather, the notice serves as an invitation to conclude discount agreements with conditions that apply equally to all contracting parties and are not dispositive, including the definition of the discount. Thus, the contract is concluded by signing the contract documents and submitting some declarations required by the health insurance funds. This formal verification is intended to ensure the reliability and performance of suppliers.
Legal remedies
In principle, the public procurement tribunal (Vergabekammer) is responsible for reviewing applications in the first instance. In the second instance, the Higher Regional Court (Vergabesenat beim Oberlandesgericht) is responsible for the immediate appeal.
The time limits for review requests are very short, 15 days after the contracting authority's failure to act on a complaint. Any ambiguities in the tender documents and the notice must be notified at the latest when submitting the tender or the request to participate, see Section 160 (3) GWB.
In addition, a bidder must give notice of defects without delay after receipt of a preliminary information (Section 134 GWB). If a bidder receives a prior information notice by electronic means and if the contracting authority maintains its intention to award the contract despite the reprimand, the application for review must generally be filed within eight days after receipt of the prior information notice; see Section 160 GWB (ten days, the procurement tribunal has to stop the awarding procedure at day nine)
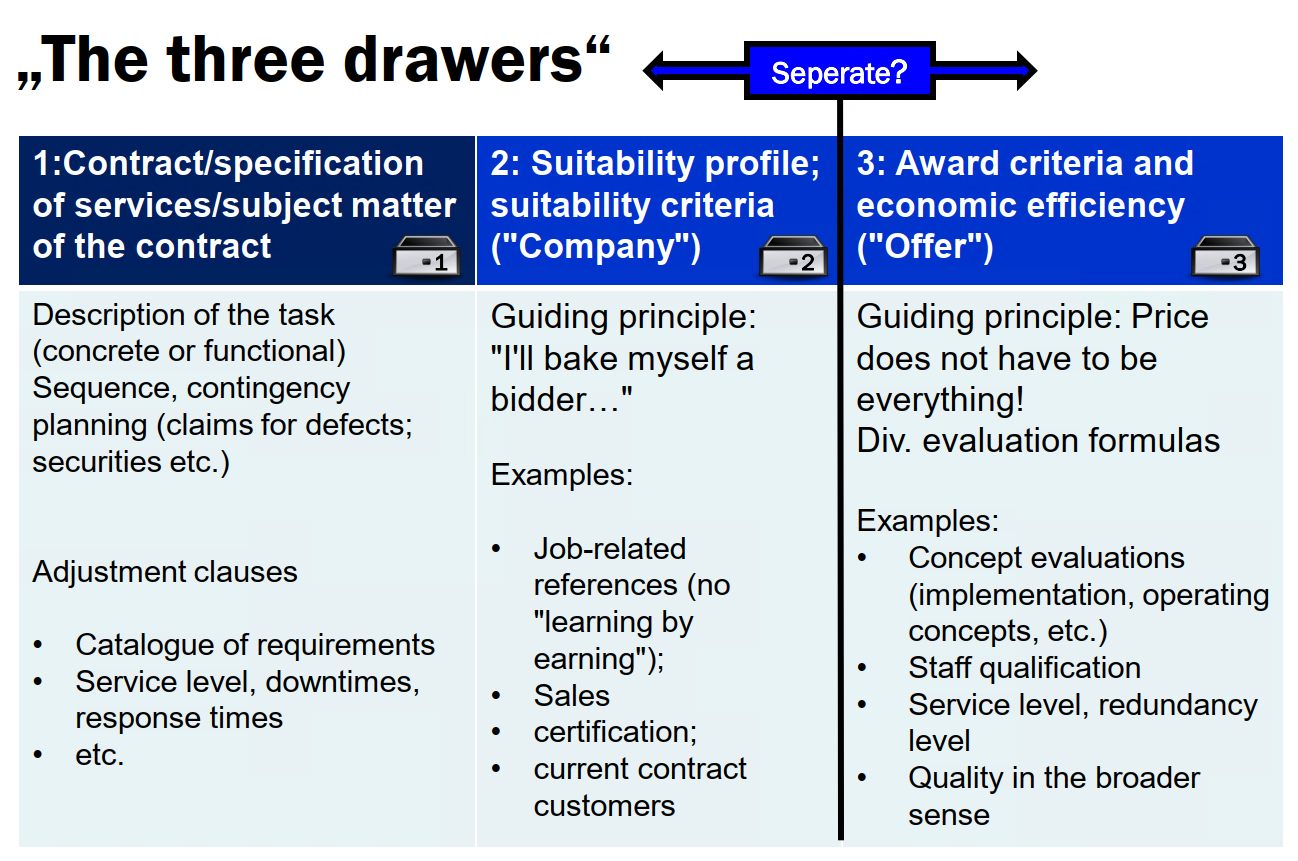
The “three drawers”
The “three drawers” are not a legal term, but a useful classification aid developed by SKW Schwarz:
- subject matter of the contract;
- suitability criteria; and
- award criteria and economic efficiency.
It can be used by public (and private) contracting authorities as well as bidders to structure procurements in order to define the parameters of the competition in a meaningful way (see graphic). The “three drawers” are also an important aid for bidders to find their way around tenders and to challenge adverse decisions made by a contracting authority.
It is important to question the extent to which a specific criterion is really needed for the procurement for each individual point. The award notice (award documentation, Section 8 VgV: Vergabedokumentation oder Vergabevermerk) is intended to serve as a control for decisions made by the contracting authority. Bidders can often draw arguments from it in review proceedings to challenge a discriminatory decision.
SKW Schwarz
4 ranked departments and 6 ranked lawyers
Learn more about the firm’s ranking in Chambers Europe 2022
View profile



